Metal-Metal Oxide Equilibria in Pure Hydrogen Atmospheres
Sep 13, 2022
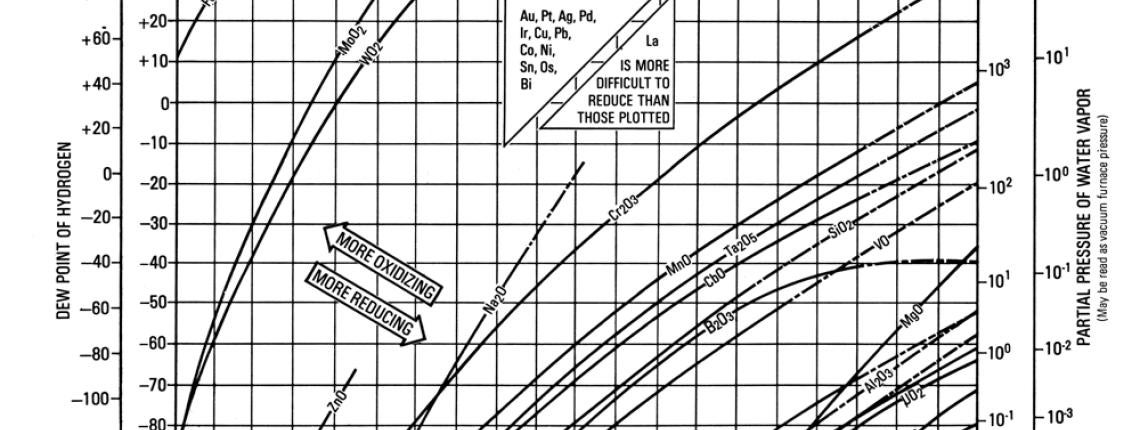
Metals oxidize due to loss of electrons on their outward facing atoms. Metals which are more reluctant to lose electrons are not easily oxidized. The application of heat can rearrange atoms causing loss of electrons, and making metals more prone to oxidation. Specific elements will rearrange to become reactive at different pressures & temperatures. The Metal-Metal Oxide Equilibria Chart maps the oxidation curve of common elements through pressures temperatures which are common for heat-treatment & brazing. This chart can be used to avoid oxidation of a metal by altering pressure & temperature.

How to Use the Chart:
If pressure and temperature are pre-determined:
- Go to the intersecting point on the chart based on pressure and temperature – this is your atmosphere.
- Verify that your atmosphere is down and to the right of curves that contain elements in your materials. This means your atmosphere is reducing, and your materials are not at risk for oxidation.
- If your material contains elements whose curve is down and to the right of your atmosphere, your material is at risk for oxidation. Solutions include increasing temperature, decreasing pressure (higher vacuum), and plating the surface to cover reactive elements.

